Your phone lights up. Your laptop hums awake. A car starts with a quiet click instead of a roar. Behind all of this lies a silent chemical engine: the battery.
Batteries don’t store electricity the way a bottle stores water. That common idea misses the real magic. A battery is a carefully controlled chemical reaction, trapped inside a small box, waiting patiently for you to press a button 🔋⚗️
Let’s open that box.
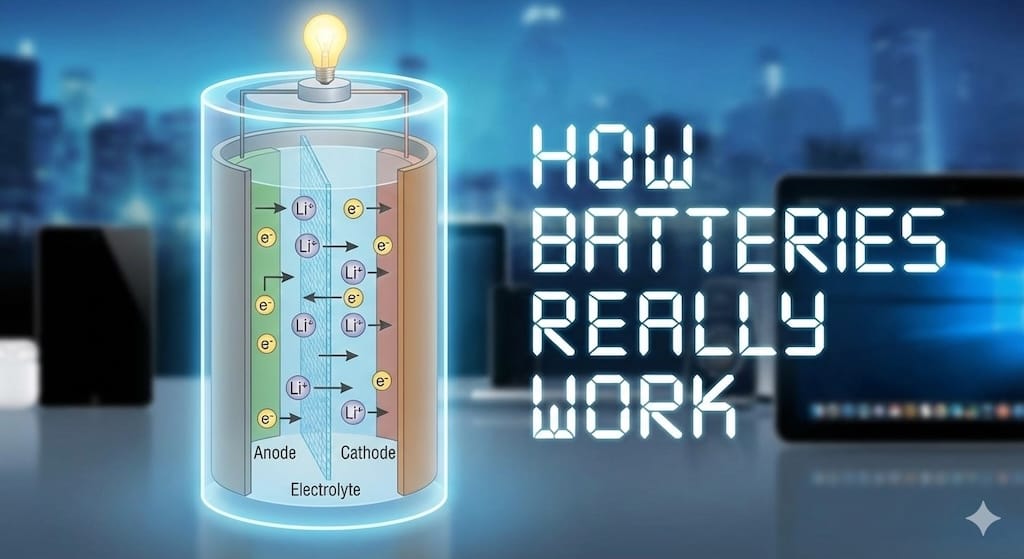
⚛️ What Is a Battery at Its Core?
At its heart, a battery is an electrochemical device. It converts chemical energy into electrical energy using predictable reactions.
Every battery, no matter how small or advanced, contains three essential parts:
- Anode – where electrons are released
- Cathode – where electrons are accepted
- Electrolyte – a medium that allows charged particles to move
When these parts interact, chemistry turns into usable power.
🔄 The Chemistry That Creates Electricity
Electricity is simply the movement of electrons. Chemistry decides when and where those electrons move.
Inside a battery:
- At the anode, a chemical reaction releases electrons
- These electrons want to reach the cathode
- A wire gives them a path to travel
- As electrons flow through the circuit, they power your device
The electrolyte keeps the reaction balanced by allowing ions to move internally while electrons travel externally.
This separation is crucial. If electrons flowed directly inside the battery, energy would be wasted as heat. Batteries force electrons to take the long way around, through your device.
🧠 Why Batteries Eventually Die
Batteries don’t run out of electricity. They run out of reactants.
Over time:
- Active chemicals are gradually used up
- Internal materials degrade
- Side reactions reduce efficiency
Once the chemical balance can no longer sustain electron flow, the battery appears “dead”.
Rechargeable batteries reverse many of these reactions, but not perfectly. Each cycle causes tiny structural changes, which is why batteries slowly lose capacity over years.
🔋 Inside a Lithium-Ion Battery (The One You Use Daily)
Lithium-ion batteries dominate modern life because lithium is unusually cooperative chemically.
Why lithium?
- It is extremely light
- It easily loses and gains electrons
- It stores a lot of energy per unit mass
Inside a lithium-ion battery:
- Lithium ions move between anode and cathode
- Electrons flow through the external circuit
- Charging pushes lithium ions back to their original side
This constant shuttle of ions is why your phone battery can be recharged hundreds of times.
🌡️ Why Batteries Heat Up
Heat is a sign of resistance.
As electrons move through materials, they encounter obstacles. This friction produces heat. Poor-quality batteries, damaged cells, or fast charging increase resistance, which increases heat.
Chemistry sets limits here. Too much heat accelerates unwanted reactions, which can damage the battery or even cause failure.
This is why battery safety is a major focus of chemical engineering.
🚗 Batteries and the Electric Vehicle Revolution
Electric vehicles are powered by chemistry, not engines.
EV batteries:
- Use thousands of individual cells
- Rely on precise chemical balancing
- Require advanced cooling systems
A single chemical imbalance can reduce range or shorten lifespan. The future of transportation depends as much on chemists as it does on engineers.
🌍 Environmental Cost of Battery Chemistry
Batteries solve many problems, but they create new ones.
- Mining lithium, cobalt, and nickel impacts ecosystems
- Improper disposal releases toxic materials
- Recycling batteries is chemically complex
Scientists are working on alternatives like sodium-ion, solid-state, and organic batteries. Chemistry again becomes the solution to chemistry’s own problems.
🧪 Why Batteries Are a Perfect Example of Chemistry
Batteries show why chemistry matters:
- Invisible reactions produce visible results
- Tiny atomic changes power massive systems
- Controlled reactions improve daily life
From wristwatches to power grids, batteries are chemistry’s most practical invention.
🧠 Why Chemistry Feels Abstract (Until It Isn’t)
You can’t see electrons moving. You can’t watch ions drifting through electrolytes. But the moment your device dies, chemistry becomes very real.
Chemistry isn’t distant or theoretical. It’s operational. It works quietly, consistently, and everywhere.
🔮 The Future of Battery Chemistry
The next breakthroughs won’t come from bigger batteries, but better chemistry:
- Faster charging without heat
- Longer lifespans with minimal degradation
- Safer materials with lower environmental cost
Every improvement begins at the atomic level.
✨ Final Thought
A battery is not a box of power. It is a carefully negotiated truce between chemicals, allowing energy to escape in a controlled stream. Every time you charge a device, you are resetting a chemical story, letting atoms prepare once more for motion.
That is chemistry. Not loud. Not flashy. Just endlessly useful 🔬🔋